Team Biotic – Nicole Cain, ND, MA
New findings explore SSRI efficacy and dysbiosis in major depressive disorder.
- A new report indicates a direct correlation between gut dysbiosis, major depressive disorder, and SSRI efficacy.
- Although the gut makes more than 90% of the body’s serotonin, gut-derived serotonin does not cross the blood-brain barrier.
- Instead, a related metabolic pathway producing kynurenine is associated with decreased serotonin, increased inflammation, and depression.
- The gut microbiota may serve as a prognostic indicator and a treatment target for major depressive disorder.
In a report scheduled for publication in the June 2023 issue of the Journal of Affective Disorders, researchers explore the association between the gut microbiome and the efficacy of selective serotonin reuptake inhibitors (SSRIs) in the treatment of major depressive disorder (MDD). Their findings indicate that an increase in specific microbial genera (Blautia spp., Coprococccus spp., and Bifidobacterium spp.) may be useful as markers predicting treatment efficacy. They also help lay to rest the notion that low serotonin is a causative factor in depression
A 2022 systematic umbrella review published in Molecular Psychiatry examined the breadth and quality of evidence behind that once-popular theory. After reviewing 17 studies involving more than 150,000 individuals, they concluded that “the main areas of serotonin research provide no consistent evidence of there being an association between serotonin (5-hydroxytryptamine, or 5-HT) and depression, and no support for the hypothesis that depression is caused by lowered serotonin activity or concentrations.” Their review also revealed evidence that long-term antidepressants may actually reduce serotonin concentration.
Serotonin Production
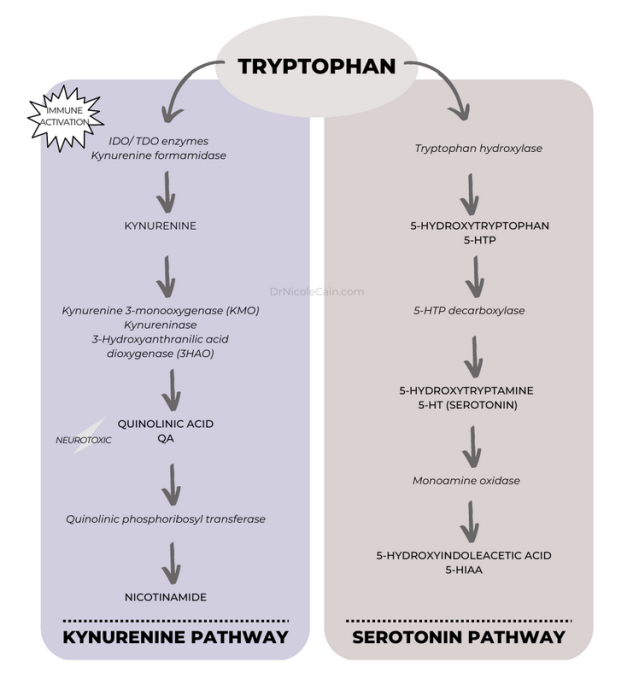
L-Tryptophan, an essential amino acid and building block of serotonin, is not produced by the body. Humans thereby rely primarily on dietary intake to provide tryptophan. The metabolism of tryptophan within the human body can follow one of two pathways, toward serotonin or toward production of kynurenine, a substance that plays a role in niacin production. Serotonin is the main focus here, but it’s noteworthy that the kynurenine pathway definitely plays a role in mood.
Tryptophan Pathways
Source: Dr. Nicole Cain
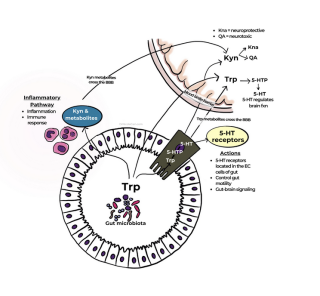
Tryptophan is metabolized into serotonin in the central nervous system by the brain’s neurons.1 It is also metabolized by specific gut microbes (e.g., Lactococcus, Lactobacillus, Streptococcus, Escherichia coli, and Klebsiella species)2 and specialized intestinal epithelial cells known as enterochromaffin cells.3 More than 90% of the body’s serotonin (5-HT) is produced in the gut. But gut-derived, peripheral serotonin typically does not cross the blood-brain barrier.4-6 Rather, it is associated with digestive activity including nutrient absorption and transport.7 Gut-derived serotonin is also involved with inflammatory response8 and glucose and lipid homeostasis,9 among other effects. A deficit of 5-HT in the gut is also associated with irritable bowel syndrome (IBS).10
Although peripheral serotonin produced by the gut cannot directly enter the brain,11 it can influence blood-brain barrier permeability.7 Furthermore, vagus nerve stimulation by the gut microbiota can alter concentration of serotonin, GABA, and glutamate within the brain—all known to influence mood.12
Additionally, peripheral serotonin itself plays a role in stimulation of the hypothalamic-pituitary-adrenal (HPA) axis, via neuroendocrine interaction.13 Notably, stress-induced hyperactivation of the HPA axis is associated with a dysregulation in the serotonergic system, including a chronic increase in the stress hormone cortisol, which is associated with both depression and suicidal ideation.14,15
The gut clearly plays a significant role in serotonin production, and peripheral serotonin does influence the brain directly and indirectly through immune function, vagal nerve stimulation, neuroendocrine feedback, and the HPA axis. But the larger question surrounding the relationship between serotonin levels in the brain and mood, specifically depression, remains unclear.
The Difference a Pathway Can Make
As shown in the illustration above, enzymes are vital to the metabolism of serotonin from L-tryptophan and its metabolite, L-5-hydroxytryptophan (5-HTP). Evidence suggests that the transport of 5-HTP across the blood-brain barrier is deficient in depressed individuals.16 As such, the most commonly prescribed antidepressant medications, SSRIs, block the serotonin transporter (SERT) in the brain, allowing more serotonin to remain in the synaptic cleft between neurons; blocking the reuptake of serotonin ostensibly keeps it active, in theory, to promote antidepressant effects.17,18 But as the 2022 review reports, the relationship between serotonin levels and depression is not clearly established.
Source: Dr. Nicole Cain
With this in mind, it’s worth taking a look at the alternative, much more common, pathway for tryptophan metabolism. In normal human physiology, more than 90% of 5-HTP is metabolized via the kynurenine pathway. But overactivation of this busy pathway in the brain results in increased production of neurotoxic compounds and lower neuroprotective compounds as well as reductions in mood-balancing neurotransmitters including dopamine, choline, and GABA.19,20
This excessive kynurenine pathway activity also happens to be correlated with exaggerated inflammation, which is itself associated with decreased levels of serotonin in the brain.21 Moreover, inflammation alone is implicated in mood disorders, with increased levels of proinflammatory proteins (cytokines) a well-established marker of major depressive disorder and treatment-resistant depression.22,23
Perhaps serotonin as a marker of depression is something of a canary in the coal mine. It may be possible that addressing serotonin reuptake between neurons is not the most efficient path toward healthy brain function, neurotransmitter optimization, and mood balance. Perhaps the root cause of depression may be better addressed with a focus on the so-called second brain: the gut microbiome.
This aligns with the findings reported in the Journal of Affective Disorders in June 2023 in which the authors conclude that the gut microbiome of patients with major depression is distinct and changes with SSRI treatment. They believe that gut dysbiosis offers a prognostic tool and therapeutic target for individuals with major depression.
References
1. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-66. doi: 10.1146/annurev.med.60.042307.110802. PMID: 19630576; PMCID: PMC5864293.
2. O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48.
3. Yano J.M. et al., Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015; 161: 264-276
4. Banskota, S., Ghia, J. E., & Khan, W. I. (2019). Serotonin in the gut: Blessing or a curse. Biochimie, 161, 56–64.
5. Yang, J., Zheng, P., Li, Y., Wu, J., Tan, X., Zhou, J., Sun, Z., Chen, X., Zhang, G., Zhang, H., Huang, Y., Chai, T., Duan, J., Liang, W., Yin, B., Lai, J., Huang, T., Du, Y., Zhang, P., … Xie, P. (2020). Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Science Advances, 6(49), eaba8555. http://dx.doi.org/10.1126/sciadv.aba8555
6. Mawe GM, Hoffman JM.. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–86.
7. Hannon, J.; Hoyer, D. Molecular biology of 5-HT receptors. Behav. Brain. Res. 2008, 195, 198–213.
8. Linden, D.R.; Chen, J.X.; Gershon, M.D.; Sharkey, K.A.; Mawe, G.M. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G207–G216
9. Linden, D.R.; Chen, J.X.; Gershon, M.D.; Sharkey, K.A.; Mawe, G.M. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G207–G216
10. Bruta, K., Vanshika, Bhasin, K. et al. The role of serotonin and diet in the prevalence of irritable bowel syndrome: a systematic review. transl med commun 6, 1 (2021). https://doi.org/10.1186/s41231-020-00081-y
11. Radjabzadeh, D., Bosch, J.A., Uitterlinden, A.G. et al. Gut microbiome-wide association study of depressive symptoms. Nat Commun 13, 7128 (2022). https://doi.org/10.1038/s41467-022-34502-3
12. Lydiard, R. B. The role of GABA in anxiety disorders. J. Clin. Psychiatry 64, 21–27 (2003)
13. Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GS, O’Rahilly S, Colmers WF, Elmquist JK, Tecott LH. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci. 2007 Jun 27;27(26):6956-64. doi: 10.1523/JNEUROSCI.2584-06.2007. PMID: 17596444; PMCID: PMC6672238.
14. Pompili M, Serafini G, Innamorati M, Möller-Leimkühler AM, Giupponi G, Girardi P, Tatarelli R, Lester D. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: a selective overview for the implications of suicide prevention. Eur Arch Psychiatry Clin Neurosci. 2010 Dec;260(8):583-600. doi: 10.1007/s00406-010-0108-z. Epub 2010 Feb 20. PMID: 20174927.
15. Correia, A.S.; Cardoso, A.; Vale, N. Highlighting Immune System and Stress in Major Depressive Disorder, Parkinson’s, and Alzheimer’s Diseases, with a Connection with Serotonin. Int. J. Mol. Sci. 2021, 22, 8525.
16. Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181.
17. Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158
18. Vahid-Ansari, F.; Albert, P.R. Rewiring of the Serotonin System in Major Depression. Front. Psychiatry 2021, 12, 2275.
19. Fukuda, K. Etiological classification of depression based on the enzymes of tryptophan metabolism. BMC Psychiatry 2014, 14, 372.
20. Wigner, P.; Czarny, P.; Synowiec, E.; Bijak, M.; Białek, K.; Talarowska, M.; Galecki, P.; Szemraj, J.; Sliwinski, T. Association between single nucleotide polymorphisms of TPH1 and TPH2 genes, and depressive disorders. J. Cell. Mol. Med. 2018, 22, 1778–1791.
21. Correia AS, Vale N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int J Mol Sci. 2022 Jul 31;23(15):8493. doi: 10.3390/ijms23158493. PMID: 35955633; PMCID: PMC9369076.
22. Hodes, G. E., Pfau, M. L., Purushothaman, I., Ahn, H. F., Golden, S. A., Christoffel, D. J., Magida, J., Brancato, A., Takahashi, A., Flanigan, M. E., Ménard, C., Aleyasin, H., Koo, H. W., Lorsch, Z. S., Feng, J., Heshmati, M., Wang, M., Turecki, G., Neve, R., … Russo, S. J. (2015). Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. The Journal of Neuroscience, 35, 16362–16376.
23. Ménard, C., Pfau, M. L., Hodes, G. E., & Russo, S. J. (2017). Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology, 42, 62–80.
